QUALITY CONTROL OF CULTURE MEDIA IN A MICROBIOLOGY LABORATORY
Shortcode UsageNew Recommendations of ESBL detection in Enterobacteriaceae in CLSI 2010
Culture media play a pivotal role in any microbiology laboratory. They are widely employed for isolation, identification and sensitivity testing of different pathogenic microorganisms. Most of the laboratories usually prepare their own media for routine diagnostics as well as research purposes. However, to ensure that the media is of good quality and capable of giving satisfactory results, proper quality management system is essential. For that purpose certain parameters of media prepared should be thoroughly checked and then passed for laboratory use.
This article is aimed to provide certain information about the parameters to be considered and the tests to be performed to ensure proper quality of media.
Requirements
Quality assurance of microbiological culture media should include tests
– to verify a satisfactory level of freedom from contamination,
– to demonstrate the correct performance of the medium when used in the usual or widely accepted manner,
– and ensure against significant physical imperfections that may compromise the utility of the media.
Performance of media listed in Table 1 should comply with expected results shown when tested according to recommended methods, including microbial strains, incubation temperature, time and atmosphere. An incubation temperature of 35 ± 2°C should be used in all cases unless otherwise specified. Incubation conditions shown in Table 1 should not be inferred to be optimal for the isolation of the respective strains from clinical material.
Each batch of media not listed in Table 1 should also be tested to demonstrate satisfactory performance and a low failure rate. A suggested minimum requirement would be the Quality Control guidelines provided by the manufacturers of Dehydrated Culture Media in their technical manuals or appropriate reference texts (e.g. Manual Clinical Microbiology).
Sterility testing should always be undertaken when media is aseptically dispensed. However, where media is terminally sterilized a protocol may be established for release on the basis of a validated sterilization process.
TABLE 1: Control Microorganisms for Growth Performance Testing of General Purpose Media for Medical Microbiology
|
Serial # |
Media |
pH (+/- 0.2) |
Method of inoculation |
Length of incubation |
Control organism |
ATCC # |
Expected result |
|---|---|---|---|---|---|---|---|
|
1 |
SBA |
7.5 |
A |
Aerobic, |
S. pneumoniae |
49619 |
Growth, α hemolysis |
|
2 |
BCNA |
7.3 |
A,B |
Aerobic, |
S. pneumoniae |
49619 |
Growth, α hemolysis |
|
3 |
MAC |
7.1 |
A,B |
Aerobic, |
P. mirabilus |
12453 |
Colourless colony, inhibition of swarming |
|
4 |
CHOC |
7.2 |
A |
CO2, |
N. gonorrhoeae |
43070 |
Growth |
|
5 |
GC |
7.2 |
A, B |
C02, 1-3days |
N. gonorrhoeae |
43070 |
Growth |
|
6 |
CLED |
7.3 |
A |
Aerobic, 1 day |
E. coli |
25922 |
Growth, yellow |
|
7 |
DNASE |
7.3 |
D |
Aerobic, 1 day |
S. aureus |
25923 |
Pink zone |
|
8 |
PPA |
7.3 |
D |
Aerobic, 1 day |
S. aureus |
25923 |
Pink colour |
|
9 |
MSA |
7.5 |
D |
1-2 days |
S. aureus |
25923 |
Colony with yellow zone |
|
10 |
BIGGY |
6.8 |
A, B |
3 days |
C. tropicalis |
13803 |
Silver metallic sheen |
|
11 |
Egg Yolk |
7.6 |
A |
1-2 days |
C. perfringenes |
3626 |
Clearness of media |
|
12 |
EMB |
7.6 |
A |
1 day |
S. enterica |
13311 |
Growth, |
|
13 |
TSI |
7.3 |
D |
1 day |
S. enterica |
13311 |
Alkalina slant, acid butt, H2S+, +/- gas |
|
14 |
CM |
7.0 |
D |
1 day |
C. histolyicum |
19401 |
Growth, |
|
15 |
CIT |
6.0 |
C/D |
1-2days |
P. aeruginosa |
27853 |
Growth, blue colour |
|
16 |
6.5% NaCl |
7.3 |
C/D |
1 day |
E. faecalis |
29212 |
Growth |
|
17 |
SS |
7.0 |
A.B |
1 day |
S. enterica |
13311 |
Colourless colonies, +/-black centers |
|
18 |
CB |
8.4 |
E |
1 day |
C. jejuni |
33291 |
Growth on subculture |
|
19 |
PSP |
7.5 |
C |
1 day |
P. aeruginosa |
27853 |
Blue green |
|
20 |
PSF |
7.5 |
C |
1 day |
P. aeruginosa |
27853 |
Yellow green |
|
21 |
OF |
7.1 |
D |
1day |
M. osloensis |
10973 |
Oxidation |
|
22 |
NB/NA |
7.4 |
A |
1day |
E. coli |
25922 |
Growth |
|
23 |
SIM |
7.3 |
D |
1day |
P. vulgaris |
8427 |
H2S+, indole+, cloudiness (motile |
|
24 |
VP |
7.2 |
C/D |
1day |
K. pneumoniae |
13883 |
Pink colour |
|
25 |
SBMHA |
7.5 |
A |
1day |
S. pneumoniae |
49619 |
Growth, α hemolysis |
|
26 |
BE |
7.0 |
D |
1-2 days |
E. faecalis |
29212 |
Growth, blackens agar |
|
27 |
HBT |
7.3 |
A, B |
1-2 days |
G. vaginalis |
14012 |
Growth, ß hemolysis |
|
28 |
CAMPY II (isolation) |
7.4 |
A, B |
2-3 days |
C. jejuni |
33291 |
Growth |
|
29 |
CAMPY (sensi) |
7.4 |
A, B |
2-3 days |
C. jejuni |
33291 |
Growth |
|
30 |
BG |
6.7 |
A, B |
1-4 days |
B. pertussis |
8467 |
Growth |
|
31 |
GLU |
7.4 |
D |
1 day |
E. coli |
22592 |
Yellow colour, |
|
32 |
LAC |
7.4 |
D |
1 day |
E. coli |
22592 |
Yellow colour, |
|
33 |
MAL |
7.4 |
D |
1 day |
E. coli |
22592 |
Yellow colour, |
|
34 |
SUC |
7.4 |
D |
1 day |
E. aerogenes |
13048 |
Yellow colour, |
|
35 |
SF |
7.0 |
A, B |
1 day |
S. enterica |
13311 |
Growth |
|
36 |
Urea |
6.8 |
D |
1 day |
P. vulgaris |
8427 |
Pink-red colour |
|
40 |
Hoyle |
7.4 |
A, B |
1 day |
C. dipetheriae |
13812 |
Grwoth, back centered colonies |
|
41 |
MHA |
7.3 |
E |
1 day |
S. aureus |
25923 |
Growth |
A: for testing nutritive properties. Dilute standard cell suspension (0.5 MF) 1:100 (0.1ml of suspension plus 9.9 ml of saline). Inoculate each medium with 10ul of dilution.
B: for testing selective properties. Dilute Dilute standard cell suspension (0.5 MF) 1:10 (0.1ml of suspension plus 9.9 ml of saline). Inoculate each medium with 10ul of dilution.
C: for testing tubed media. Inoculate each medium with 10ul of the standard cell suspension (0.5 MF). Adjust the inoculum if lighter or heavier growth is required.
D: for testing biochemical media. Inoculate each medium according to the procedure for the routine use of the medium.
E: for testing transport media.
Raw material parameters
The quality of the media depends directly upon the quality of the raw materials used for their preparation.
-
Water is the most important raw material
-
The parameters to be checked are presence of copper ions, conductivity and pH.
-
Ideally there should be no copper ions present in water because it is inhibitory for the growth of microorganisms.
-
The conductivity should be less than 15 μS (microsiemens).
-
The pH of the water should be slightly on the acidic side, but should not be less than 5.5.
-
The quality of petri dishes used for pouring of media is also an important factor. Normally petri dishes are ethylene oxide (EtO) sterilized or gamma irradiated. If EtO sterilized they should be then checked for residual EtO toxicity, as this may affect the growth of the microorganisms. The maximum permissible limit for residual EtO is 1 μg/g and it can be measured by standard gas chromatographic methods.2
-
Only borosilicate glassware should be used because soda glass can leach alkali into the media.
-
Various additives used in preparation of media. Blood is the most important one of them. The sterility, homogeneity, viscosity and colour of the blood should be scrupulously checked before it is used for media preparation. For other additives the certificate of analysis and sterility conditions should be considered
Sterilization parameter
Sterilization of the media plays an important role in the quality of the media.
-
Generally autoclaving is carried out for sterilizing the media. However, the time of autoclaving and the quantity of media sterilized should be closely regulated.
-
Heat treatment of complex culture media may result in its nutrient destruction either by direct thermal degradation or by reactions between the components. Therefore, it is very important to optimize the heating process to minimize heating damages.
-
The suggested cycle is stage 1:20-121°C, stage 2: <100-121°C, stage 3:121-121°C and stage 4:121-80°C.
-
The volume of the media in one sterilization batch should be kept small, ideally two liters.
-
Regular checking of the sterilization process by indicators should be done; temperature and pressure should also be constantly monitored. Sterilization indicators such as biological indicators and Bowie Dick test are available to check the efficiency of the process. Biological indicators such as spores of Bacillus stearothermophilus can be used to check the spore killing efficacy.
Physical parameters
-
Media prepared should be screened for physical characteristics such as excessive bubbles or pits, unequal filling of plates (uniform leveling), cracked medium in plate and freezing or crystallization.
-
All the above mentioned characters can be checked visually by naked eye. However, for unequal filling of plates, thickness of medium can be checked at four points. These four points are the two ends of the two diameters of the plate, which are at right angles to each other. Thus all the four sides can be simultaneously checked. The thickness at the four points is noted down and the mean thickness is determined and reported as mean thickness of the medium in the plate, which must be 4.0 ± 0.2 mm.
-
The pH value of the medium is also one of the important physical characters, which must be checked. It can be measured while preparation of the medium before and after autoclaving by using the standard pH meter after proper calibration with standard buffers.
Microbiological parameters
- Growth supporting characteristics is the most important parameter while conducting quality control of media.
- Standard inoculating procedures should be used.
- The results should be examined both qualitatively and quantitatively and while testing new lots, both previous batch and new batch should be simultaneously grown.
- CLSI has laid down certain guidelines for the control organisms to be used for every medium, the desired inoculum concentration and their expected growth results.
- Inoculum for every medium can be prepared according to the following method:
The control organism is inoculated in soyabean casein digest (SCD) broth and incubated for 4 hours to get a cell density comparable to 0.5 McFarland’s standards. The standard suspension should give a colony count of 107- 108 cfu/mL (0.08-0.1 absorbance at 625nm). A 10 μL quantity of inoculum of 1 in 10 and 1 in 100 dilution in normal saline or in SCD broth should be used for selective and nonselective media respectively.
These diluted inocula are used to ascertain the growth supporting capacity of the media. The inoculation is done in duplicates for each type of inoculum. After inoculation, the plates are incubated at 37°C for 24 hours and their growth and colony characteristics are observed. The results can be reported by mentioning presence or absence of growth and the growth characteristics in a tabular form.
In practice, the absolute measurements of growth of microorganisms are either time consuming or require sophisticated instruments. Colony size may be used to see the performance but it is again an insensitive indicator.
Colony characteristics are subjective and very difficult to record. Methods like ecometric method and proportional method, which gives us comparative data, are therefore suitable for routine quality control of microbiological performance of culture media. They can be utilized to check both the growth as well as inhibition characteristics of media.
Ecometric method
-
This is a simple and numerical method. Both absolute growth index (AGI) and relative growth index (RGI) can be determined.
-
The method is based on streaking of inoculum to extinction. This method can be used to compare results with previous batches of same medium or between selective and nonselective media. In this method five millilitre of brain heart infusion broth is noculated with loopful of chosen test organism and incubated for four hours.
-
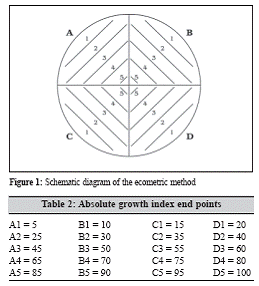
The petri dish of given medium should be divided into 4 quadrants and streaking lines should be drawn on them as shown in figure 1. One microlitre loop has to be charged with culture and streaked in the way as A1, B1, C1, D1, A2, B2 …… upto D5 without flaming or recharging the loop.
The procedure has to be repeated for the control plate. After incubation the last point should be noted in both test and control on which growth occurs. These are the end points of test and control media. These readings can be used to calculate the absolute growth index (AGI) and relative growth index (RGI) of the medium. The AGI is obtained by noting the end points (Table 2).
The RGI is a comparison of the AGI of the test plate and control plate.
![]()
The RGI varies according to the intended purpose of medium. In order to check efficiency the RGI should be close to 100%, whereas for checking inhibition it should be closer to 0%. The performance of a selective agar can be tested using an organism that has been selected to check isolation and using another that has been selected to check inhibition.
Productivity ratio
Determining the productivity ratio (PR) of a medium is another method of determining the performance relative to the control medium, which should be a nutritious agar like tryptone soy agar (TSA). The inoculum used must be same for both media and PR is calculated by counting the colonies on test and control media.
![]()
The method follows the modified Miles & Misra technique. A tenfold dilution of an overnight culture of test organism is prepared in buffered peptone water. The test plates are divided into 4 quadrants and each quadrant is marked with the dilution to be used as shown in figure 2. Starting with highest dilution, each drop of the dilutions is placed on the relevant quadrant. Same step is repeated for control plate. Each drop is spread in the corresponding quadrant and incubated at 37°C for 18 hours. The colonies of the lowest dilution are counted for both test and control plates.
Contamination parameter
This is a very crucial parameter for the determination of the quality of media. The batch must be scrupulously checked for contamination before passing for laboratory use. It is also suggested that the whole batch of the prepared media be checked for contamination by keeping the plates at least for three days at room temperature. Alternatively, two plates from the test batch can be taken and placed into the incubator set at 37°C for 24 hours. After required incubation, the plates are checked for any growth. If there is any growth, the process is repeated, taking again two plates from the same batch. If contamination occurs again, it is inferred that contamination has occurred in the prepared batch. As per recommendations more than 10% contamination requires the batch to be discarded.
Gel strength parameter
Gel strength is an indication of level of solidification of the agar in the medium. The gel strength is measured by using a tripod stand with a central rod that is used to impart pressure on the agar. The lower end of the rod has a spherical portion, which rests on the medium surface. The upper end of the rod has a platform on which standard weights are placed. The spherical portion of the central rod is placed on the medium and weights are placed on the upper platform one by one and observed for some time. The process is continued until the agar breaks under the weight of the central rod. While calculating the gel strength the weight of the central rod should be deducted. The force imparted by the rod on the agar surface can be calculated by the formula:
![]()
Where, w = weight kept on the platform, r = radius of the spherical portion at the lower end of the central rod and π= 3.14.
A gel strength of about 300 – 500 dynes/cm2 will give satisfactory results.
Trouble shooting guide
Table 3 gives information about the various problems found in culture media and their probable causes.
Table 3: Trouble shooting guide for culture media
|
Soft Gel |
Excess heat, pH too low causing acid |
|
pH incorrect |
Contaminated glassware, impure water, |
|
Abnormal colour |
Impure water, dirty glassware, |
|
Darkening |
Excess heat, deterioration of dehydrated |
|
Precipitation |
Excess heat, deterioration of dehydrated |
|
Toxicity |
Excess heat (scorching or burning), |
|
Poor growth |
Contaminated water or glassware, |
|
Poor selective |
Contaminated water or glassware, |
References:
1. Quality control of culture media in a microbiology laboratory. S Basu, A Pal, PK Desai
Indian Journal of Medical Microbiology, (2005) 23 (3):159-163
2. Guidelines for Assuring Quality of Medical Microbiological Culture Media
Media Quality Control Special Interest Group, Australian Society for Microbiology
ASM Approved Guidelines – September 1996
Dr summiya nizamuddin

